One of the most common attributes of chemical materials that we observe and feel on a daily basis is the density of materials. One of the things we notice in the structures of atoms, is that the atom is mostly space, with a small heavy nucleus and very light electrons orbiting the nucleus. So, how heavy something feels is related to how many protons and neutrons are in the nucleus of atoms that make up molecules. For example, aluminum is much lighter than iron. The "heaviness" of a material is quantified through a characteristic called density.
For this activity, and future ones, we will introduce the usage of simulations and gaming to aid in our understanding of chemical principles. The simulation package we will utilize can be found at this site:
http://phet.colorado.edu/
To complete Activity 5, complete the tasks below:
1. Run the Build an Atom simulation http://phet.colorado.edu/en/simulation/build-an-atom and build a neutral lithium atom and a neutral boron atom. Take a picture, or a screen shot, of these two atoms and place them on your blog. List the number of protons, neutrons and electrons for each. Also look up and post the density for each of the elements on your blog.
Lithium:
3 Protons
3 Neutrons
3 Electrons
Density: 0.534 grams per cubic centimeter
Boron:
5 Protons
5 Neutrons
5 Electrons
Density 2.37 grams per cubic centimeter
2. Define density and the equation for density and post on your blog.
DENSITY is a physical property of matter, as each element and compound has a unique density associated with it. Density defined in a qualitative manner as the measure of the relative "heaviness" of objects with a constant volume.
The formula for density is 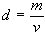
d = density
m = mass
v = volume
3. Run the Density simulation http://phet.colorado.edu/en/simulation/density and complete one(your choice) of the prepared Teaching Ideas and post your results on your blog. The activity you choose should be one of the student intended activities.
Teaching Idea Used: Density and Bouyancy ; Created by: Milton Johnson
PhET- Density
Activity- Funsheet
Custom Section
|
Material
|
Mass (kg)
|
Volume (L)
|
Density (kg/L)
|
Does it Float?
|
|
Styrofoam
|
.75
|
5.00
|
.15
|
yes
|
|
Wood
|
2.00
|
5.00
|
.4
|
yes
|
|
Ice
|
4.60
|
5.00
|
.92
|
yes
|
|
Brick
|
10.00
|
5.00
|
2
|
no
|
|
Aluminum
|
13.50
|
5.00
|
2.7
|
no
|
1.
In the custom setting, choose the ‘My Object’ option
in the material drop down box. Set the
mass of your object to 4 kg. Adjust the volume
to find the minimum volume needed to make the object float.
Volume 4.01L Density 1.00(kg/L)
2. How does the density of a large piece of aluminum compare
to a small piece?
·
There is the same amount of density in a large
piece of aluminum when compared to a smaller piece of aluminum.
Same Mass Section
|
Material
|
Mass (kg)
|
Volume (L)
|
Density (kg/L)
|
Does it Float?
|
|
Blue
|
5.00
|
5.00
|
1
|
no
|
|
Yellow
|
5.00
|
5.00
|
1
|
yes
|
|
Green
|
5.00
|
2.50
|
2
|
no
|
|
Red
|
5.00
|
1.25
|
4
|
no
|
Same Volume Section
|
Material
|
Mass (kg)
|
Volume (L)
|
Density (kg/L)
|
Does it Float?
|
|
Blue
|
6.00
|
5.00
|
1.2
|
no
|
|
Yellow
|
8.00
|
5.00
|
1.6
|
no
|
|
Green
|
4.00
|
5.00
|
.8
|
Yes
|
|
Red
|
2.00
|
5.00
|
.4
|
yes
|
3. Looking at the
data on the previous page, what must be true about the density of
an object in
order for it to float?
·
The density must be 1(kg/L) or below in order to
float.
Same Density Section:
4. Calculate the
density of the blue object in this section.
Mass 3.00kg Volume 3.00L Density 1(kg/L)
5. Explain why both
the yellow and red objects float when they have different sizes.
·
They both have the density of 1(kg/L)
Mystery Section:
6. Before you start,
pick an object that you think will float.
D
Pick an object
that you think will sink. E
|
Material
|
Mass (kg)
|
Volume (L)
|
Density (kg/L)
|
Does it Float?
|
|
A
|
65.14
|
3.38
|
19.27
|
no
|
|
B
|
.64
|
.64
|
1
|
yes
|
|
C
|
4.08
|
4.08
|
1
|
yes
|
|
D
|
3.10
|
3.10
|
1
|
yes
|
|
E
|
3.53
|
1.00
|
3.53
|
no
|
7. In the Custom
section describe the difference between how Styrofoam and ice
floated. Also explain why you think this is the case?
·
Styrofoam floated with the majority of the block
above the water, where the Ice block only had a little bit of ice above the
water. This could be because of the difference in the amount of density each
item has. The less dense the item, the more it floats.
8. In the Same Mass
Section discuss what was interesting about the blue object’s behavior in the
water.
·
Even though the Blue object had the same amount
of mass as volume and a density of 1, it did not float unlike a similar object
that had the same mass, volume, and density.
9. In the Mystery
Section, click on the “Show Table” button.
What is the most dense
object on the
list? Write its density as well.
·
Gold with a density of 19.3
10. List something
you learned from this activity.
·
Even though an object appears smaller that does
not mean that it will float.
·
Objects of different materials may different
densities even though they are of the same mass or volume.
·
The majority of objects will float if they have
a density of 1(kg/L) or lower.
4. Complete the Mystery Blocks activity on the Density simulation. Post on your blog the data you collected (mass, volume, and density) and the identification of the material and the known density.
|
Material
|
Mass (kg)
|
Volume (L)
|
Density (kg/L)
|
Material
|
Known Density (kg/L)
|
|
A
|
65.14
|
3.38
|
19.27
|
Gold
|
.40
|
|
B
|
.64
|
.64
|
1
|
Water
|
1.00
|
|
C
|
4.08
|
4.08
|
1
|
Gasoline
|
.70
|
|
D
|
3.10
|
3.10
|
1
|
Ice
|
.92
|
|
E
|
3.53
|
1.00
|
3.53
|
Diamond
|
3.53
|
5. Identify and post on your blog the Science Standards that could be met through these activities completed in Activity 5
Based on the Science Standards examined in Activity 4, I think that the following standards would fit this activity:
- A.4.2 When faced with a science-related problem, decide what evidence, models, or explanations previously studied can be used to better understand what is happening now
- A.4.3 When investigating a science-related problem, decide what data can be collected to determine the most useful explanations
- C.4.2 Use the science content being learned to ask questions, plan investigations, make observations, make predictions, and offer explanations
- C.4.4 Use simple science equipment safely and effectively, including rulers, balances, graduated cylinders, hand lenses, thermometers, and computers, to collect data relevant to questions and investigations
- C.4.5 Use data they have collected to develop explanations and answer questions generated by investigations
- C.4.6 Communicate the results of their investigations in ways their audiences will understand by using charts, graphs, drawings, written descriptions, and various other means, to display their answers
- C.4.7 Support their conclusions with logical arguments


No comments:
Post a Comment